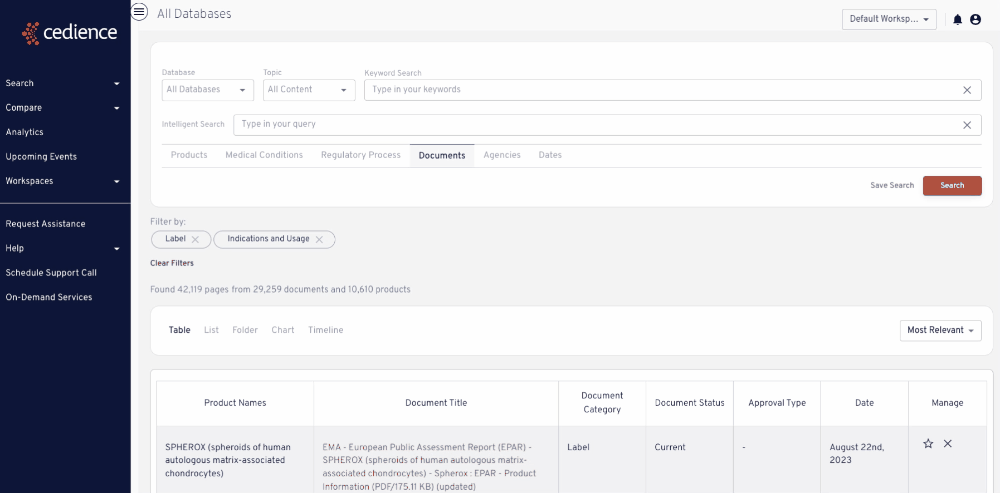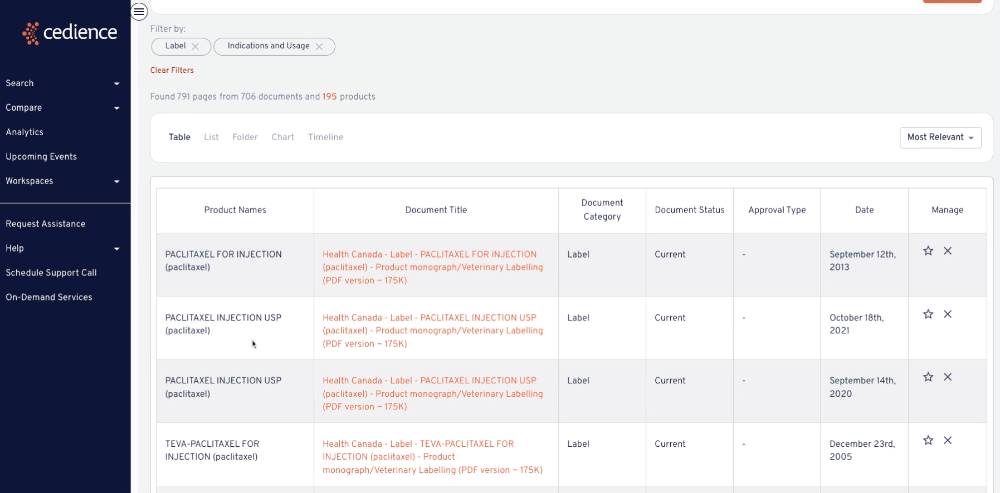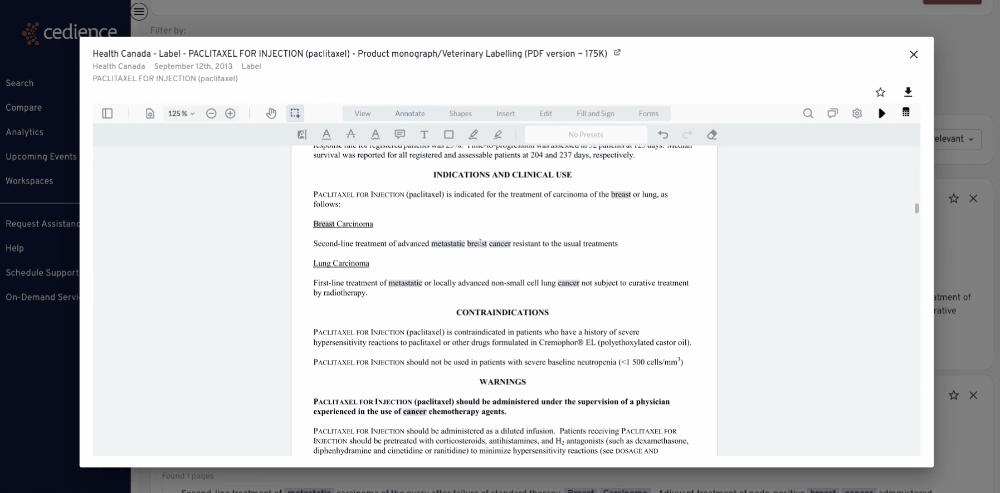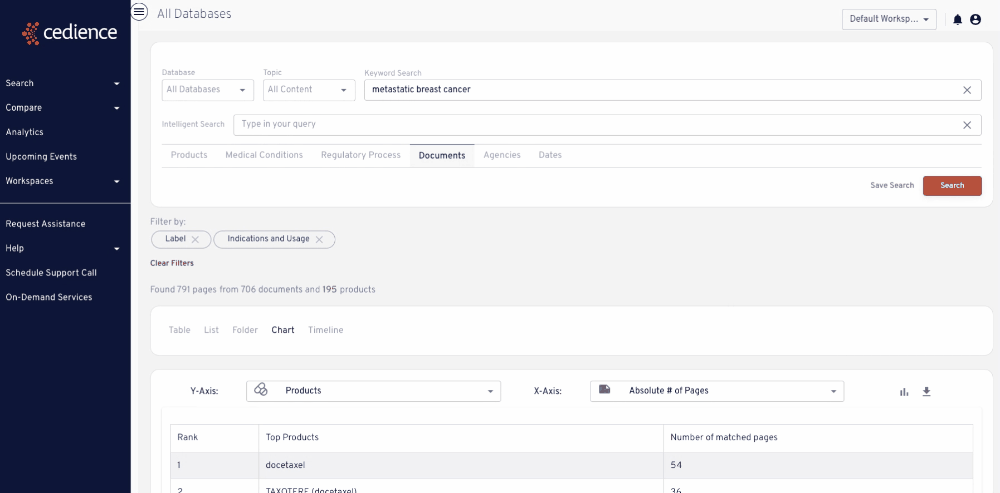
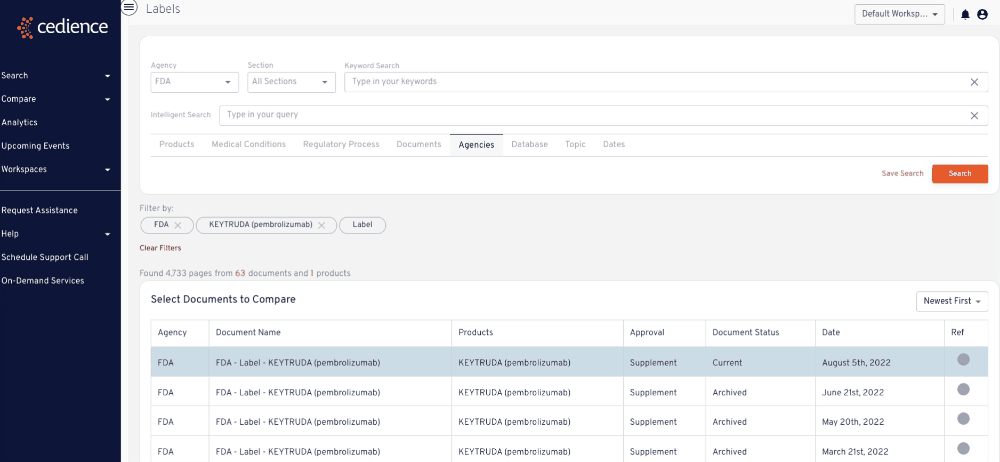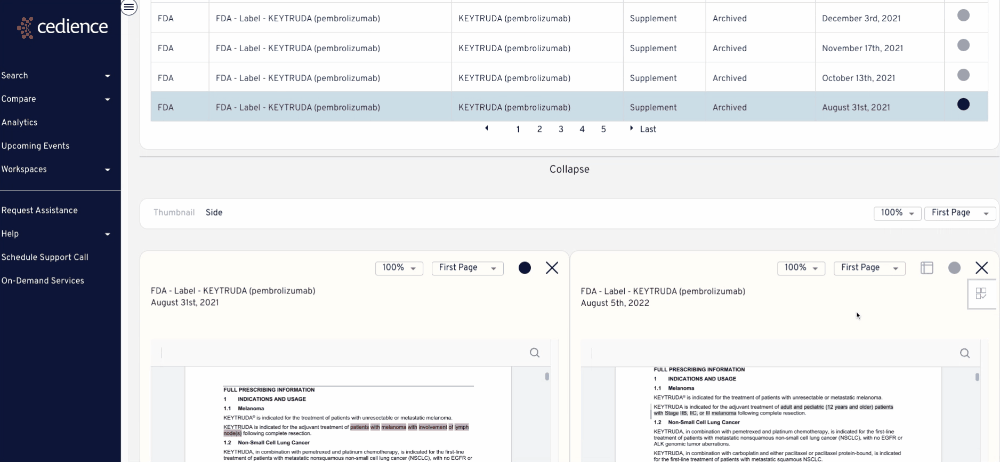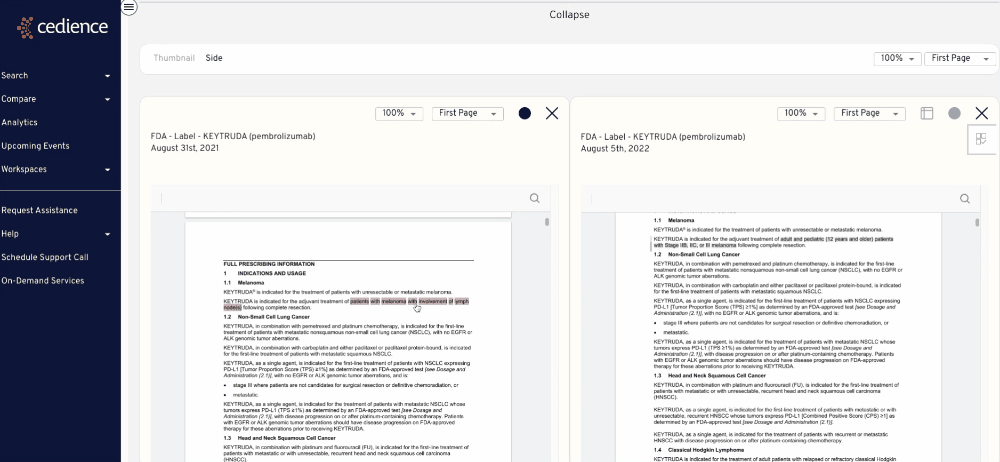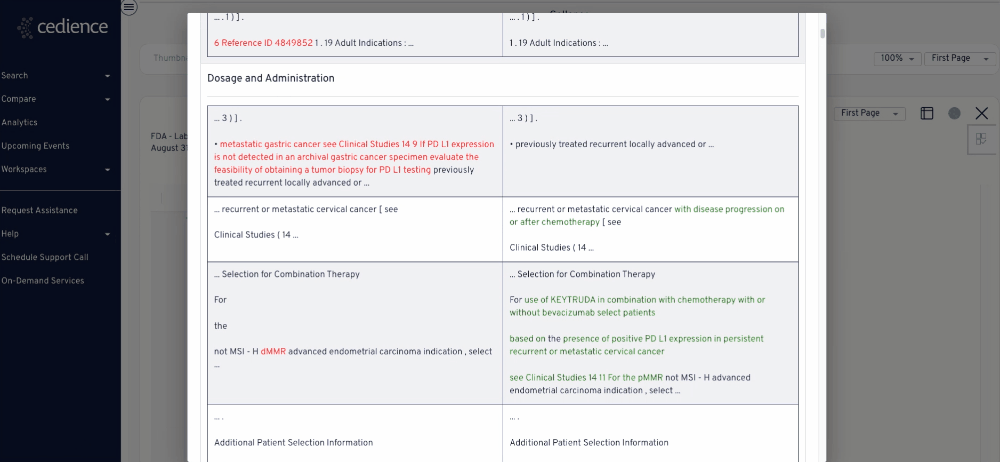
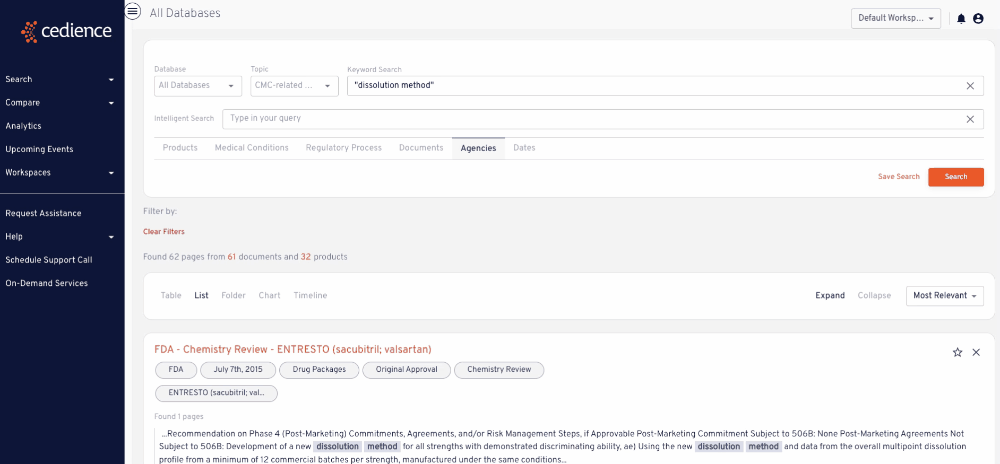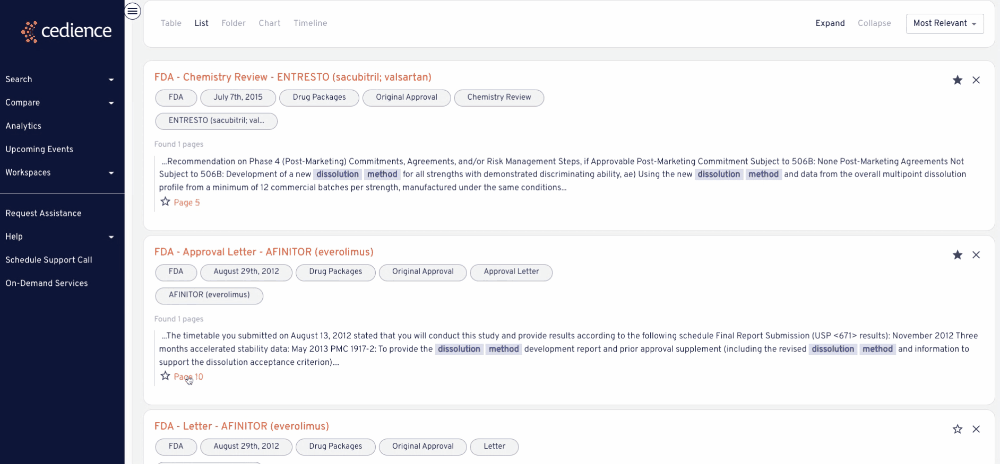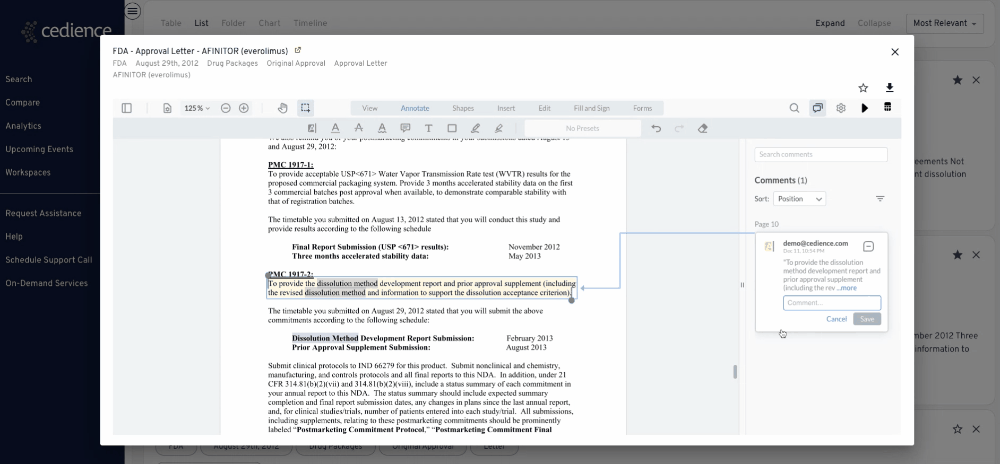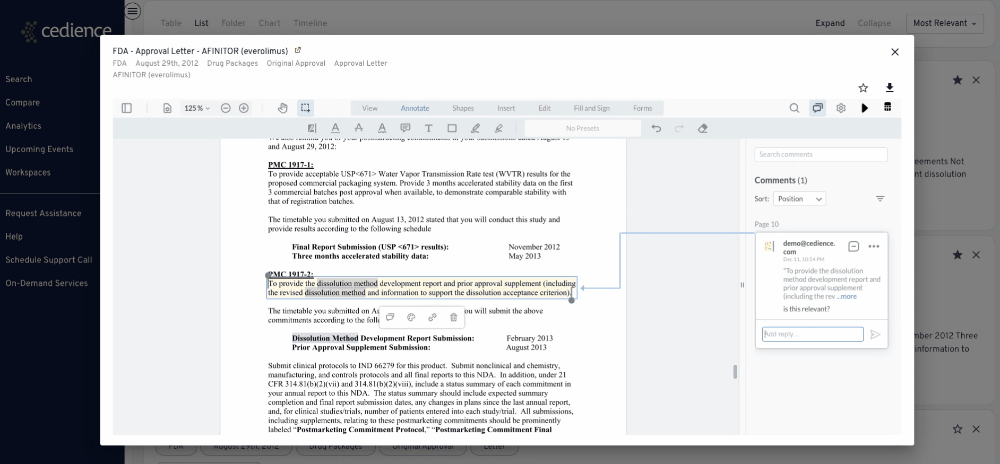
Reconstruct development timelines
Quickly piece together the detailed development timelines of relevant products without having to sift through thousands of documents. Visualize the studies that were submitted and the events leading up to regulatory decisions.
Learn from the precedents
The impracticality of sifting through an abundance of scientific knowledge means there's a huge amount of redundancy in drug research and development. Utilizing our platform means you can learn from past development strategies and leverage the work of your peers, including their wins and mistakes.


Learn from the precedents
The impracticality of sifting through an abundance of scientific knowledge means there's a huge amount of redundancy in drug research and development. Utilizing our platform means you can learn from past development strategies and leverage the work of your peers, including their wins and mistakes, not just your own.

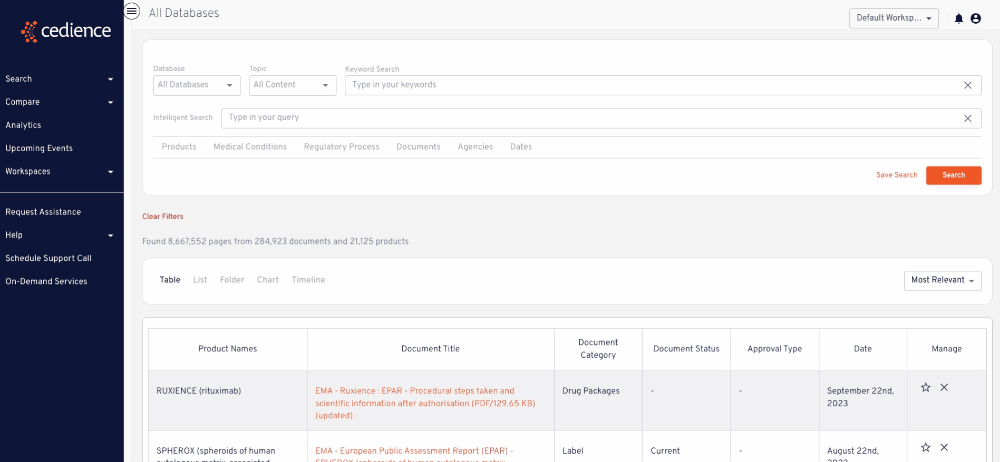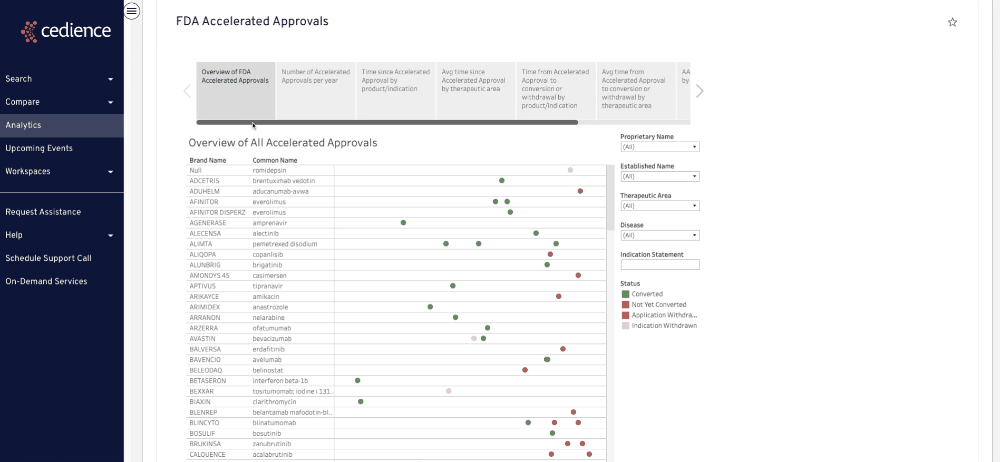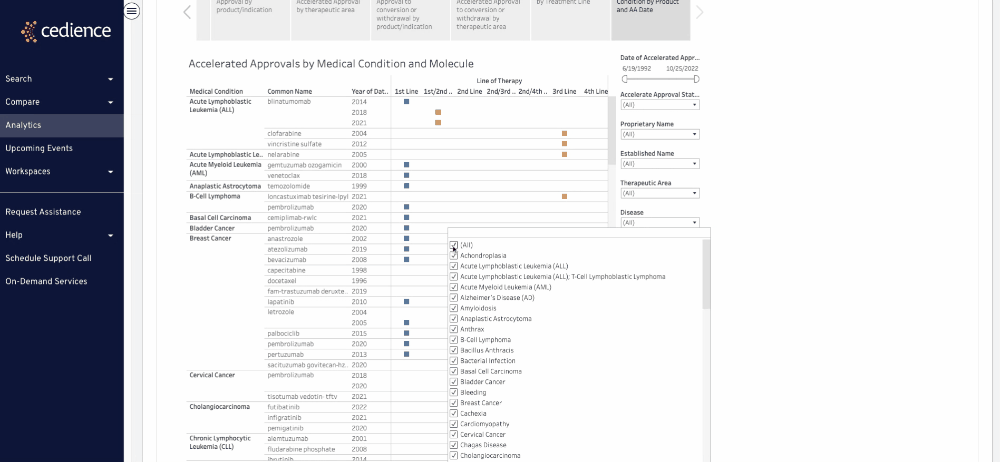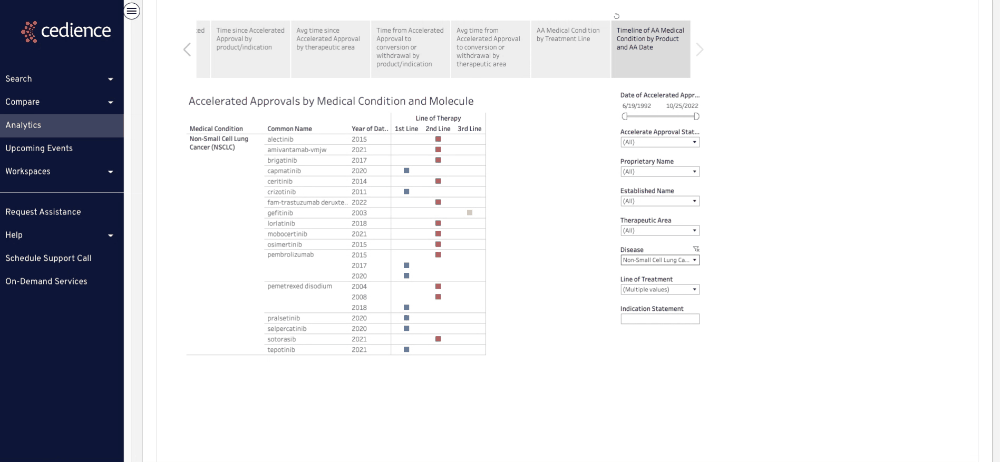
Fill intelligence gaps
Many questions on past approvals remain unanswered due to their complexity. The information in the regulatory databases is disconnected, making it impossible to see the full picture. Our technology helps answer the most complex questions to equip you with all the details you need to make a decision.
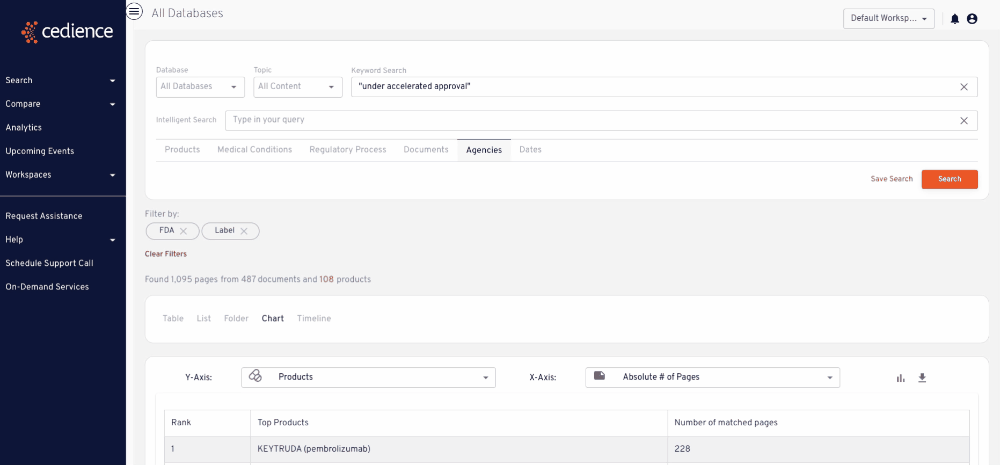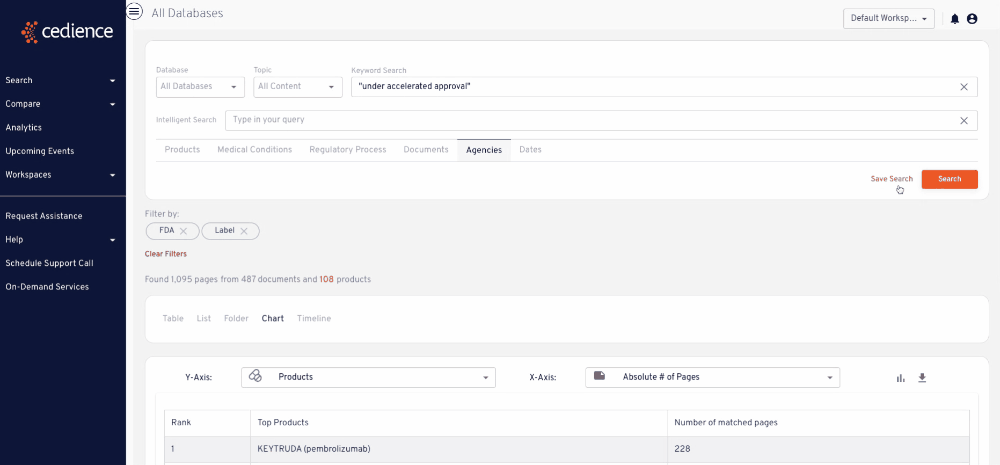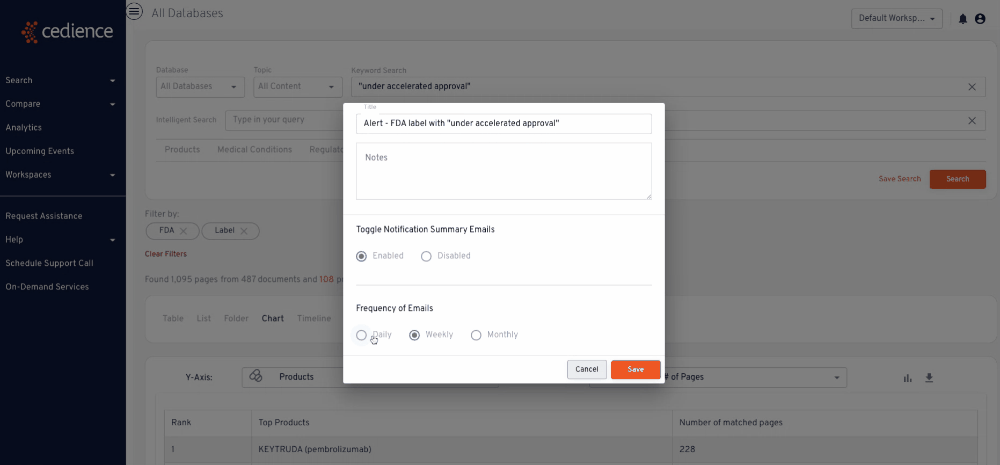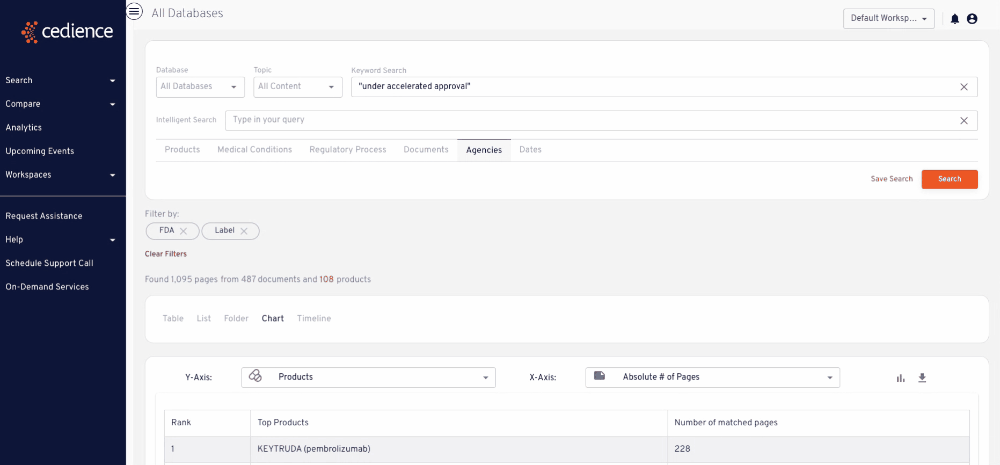
Stay up to date on topics of interest
The information critical to your decisions is hidden in an overload of alerts and newsletters. Regulatory intelligence teams rely on our platform to detect and monitor key topics of interest wherever they arise across the regulatory knowledge bases.


Stay up to dates on topics of interest
The information critical to your decisions is hidden in an overload of alerts and newsletters. Regulatory intelligence teams rely on our platform to detect and monitor key topics of interest wherever they arise across the regulatory knowledge bases.